Procedures and quality controls :
TEV protease :
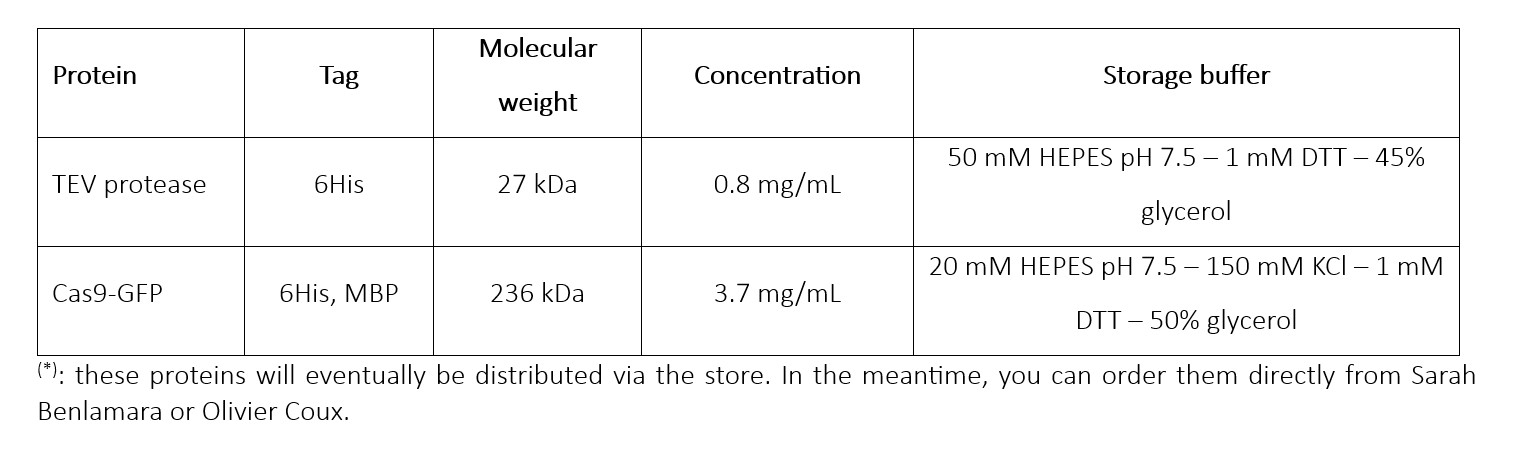
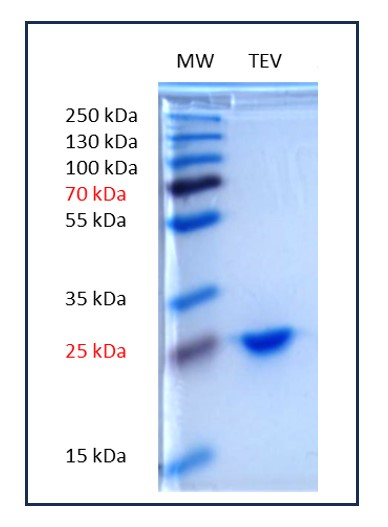
The protease was expressed using the 6His-pTEV plasmid (backbone pET28 - map available on request), in the E.coli Rosetta pLysS strain. It was then affinity purified on a Ni-NTA column, followed by anion exchange after desalting. The pure fraction was centrifuged to remove aggregates and stored in 50% glycerol.
Its activity was then tested using Cas9-GFP, which features a TEV site between the 6H-MBP tag and Cas9-GFP.
Cleavage efficiency depends on the accessibility of the enzyme binding site and can therefore vary a lot from one substrate to another. We recommend adapting the amount of TEV to the substrate, using a range of decreasing amounts of TEV starting at 1 µg TEV for 1 µg substrate.
Purity analysis (Coomassie – 10% acrylamide) :
Cas9-GFP
The protein was expressed from the 6His-MBP-Cas9-GFP construct (addgene pMJ922) in the E.coli Rosetta pLysS strain. It was purified via the following steps:
- Affinity on Ni-NTA column
- Cation exchange on MonoS column
- Gel filtration on Superdex 200 column.It was then concentrated (Vivaspin) to 3.7 mg/mL
Purity analysis (Coomassie – 8% acrylamide) : ➜ 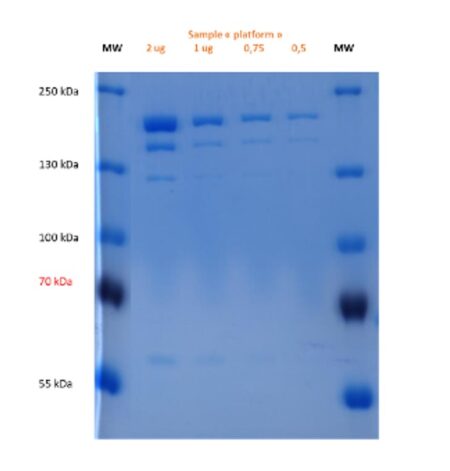
Activity analysis :
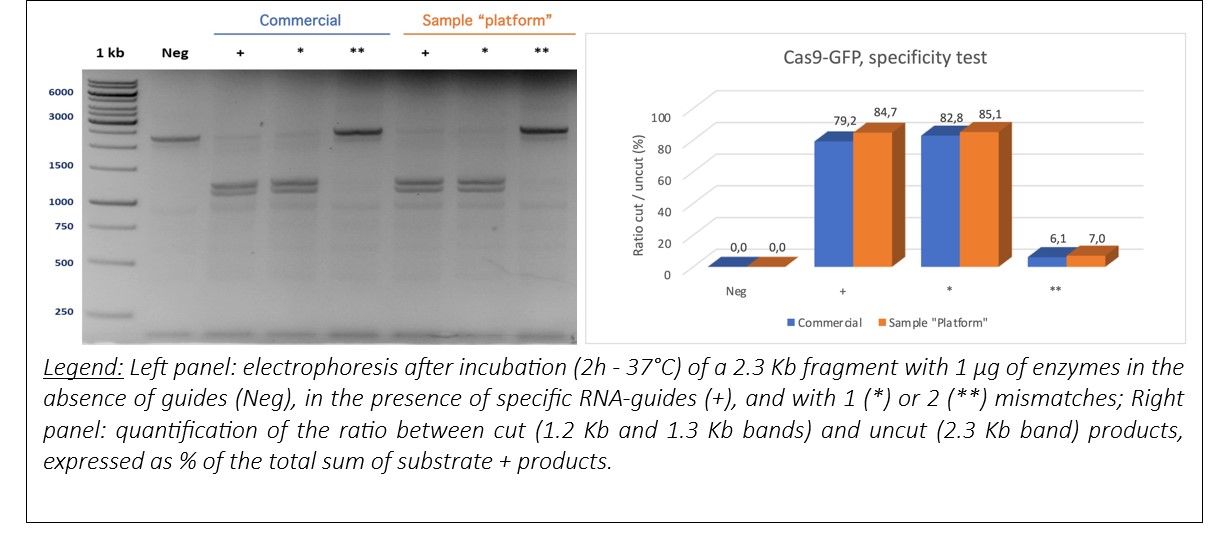
The activity of Cas9-GFP was tested in vitro by comparing it with a commercial Cas9. Two tests were carried out: an efficacy test and a specificity test. The sample produced by the platform gave comparable results to the commercial enzyme.
Efficacy :
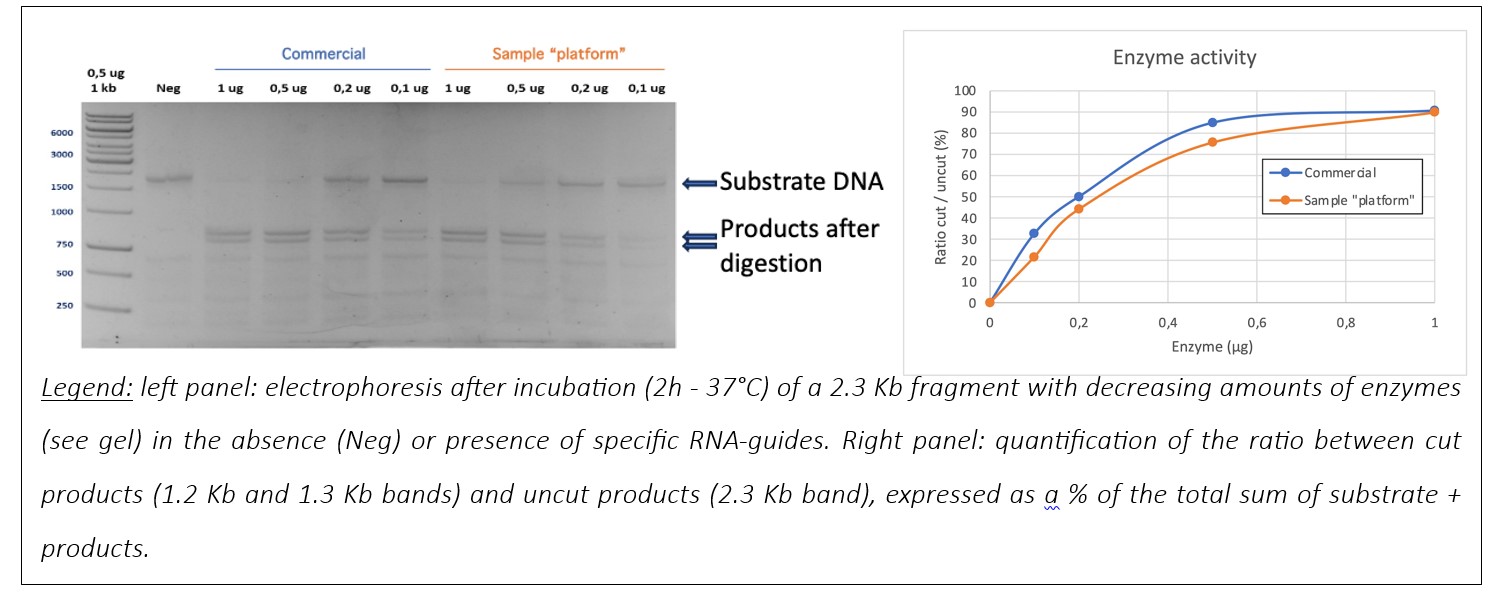
Specificity :