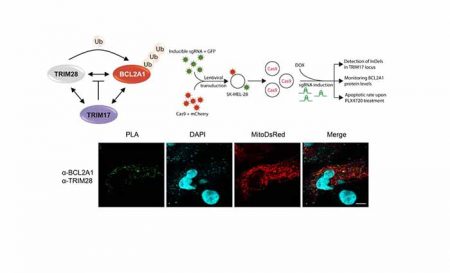
Failure to eliminate cancer cells by apoptosis is crucial in tumor development. Therefore, the key approach to achieving successful antitumor response is to harness strategies allowing the elimination of pro-survival factors to the tumor. BCL2A1 is involved in chemoresistance of melanoma and is not targeted by BH3-mimetic inhibitors. In this study Lionnard et al reveal that TRIM28 and TRIM17 are core factors of a ‘molecular rheostat’ that regulate the ubiquitination and anti-apoptotic activity of BCL2A1 in melanoma cells. Moreover, we showed that GSK3 is involved in the phosphorylation-mediated inhibition of BCL2A1 degradation. These results highlight the role of targeting factors that control BCL2A1 steady state in breaking BCL2A1 protective function to kill melanoma cells.
Lionnard L, Duc P, Brennan MS, Kueh AJ, Pal M, Guardia F, Mojsa B, Damiano MA, Mora S, Lassot I, Ravichandran R, Cochet C, Aouacheria A, Potts PR, Herold MJ, Desagher S, Kucharczak J.
TRIM17 and TRIM28 antagonistically regulate the ubiquitination and anti-apoptotic activity of BCL2A1 (S. Desagher group).
Cell Death Differ. 2018 Jul 24. doi: 10.1038/s41418-018-0169-5.