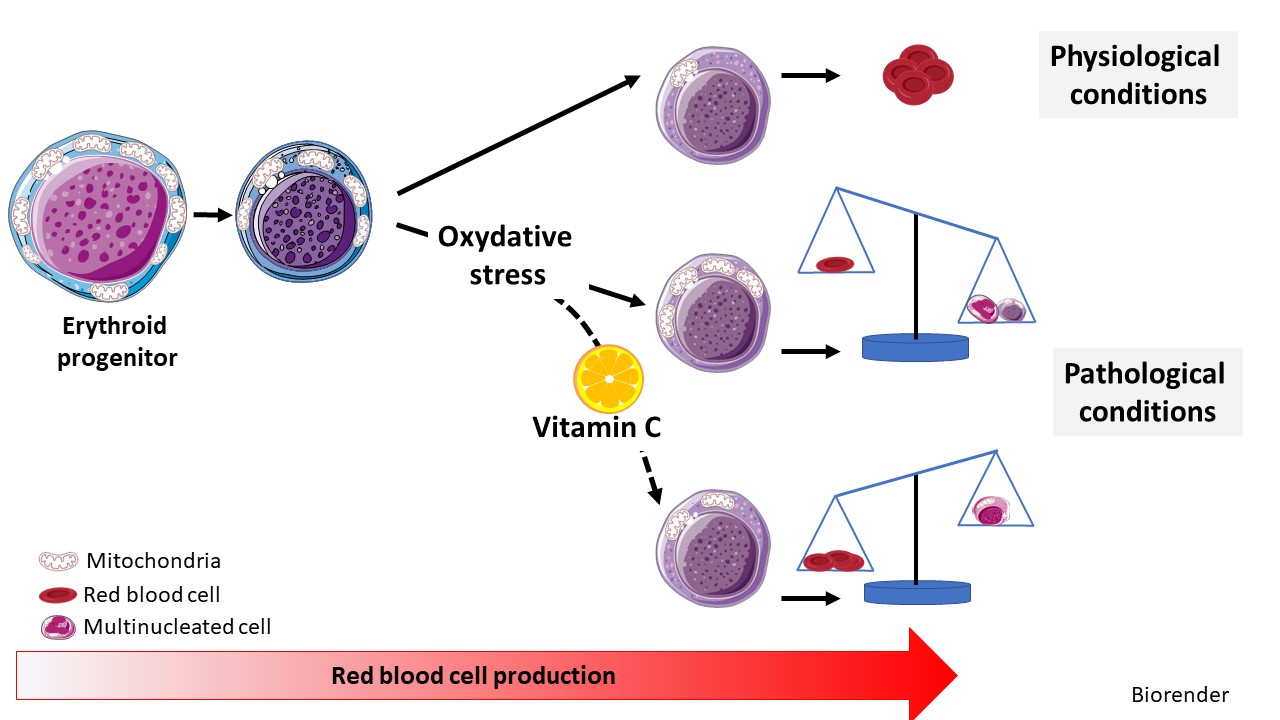
The metabolic changes controlling the stepwise differentiation of human stem and progenitor cells (HSPCs) to mature erythrocytes are poorly understood. Here, we show that HSPC development to an erythroid committed proerythroblast results in augmented glutaminolysis, generating alpha-ketoglutarate (aKG) and driving mitochondrial oxidative phosphorylation (OXPHOS). However, sequential late-stage erythropoiesis is dependent on decreasing aKG-driven OXPHOS, and we find that isocitrate dehydrogenase (IDH1) plays a central role in this process. IDH1 downregulation augments mitochondrial oxidation of aKG and inhibits reticulocyte generation. Furthermore, IDH1 knockdown results in the generation of multinucleated erythroblasts, a morphological abnormality characteristic of myelodysplastic syndrome and congenital dyserythropoietic anemia. We identify vitamin C homeostasis as a critical regulator of ineffective erythropoiesis; oxidized ascorbate increases mitochondrial superoxide and significantly exacerbates the abnormal erythroblast phenotype of IDH1-downregulated progenitors, whereas vitamin C, scavenging reactive oxygen species (ROS) and reprogramming mitochondrial metabolism, rescues erythropoiesis. Thus, an IDH1-vitamin C crosstalk controls terminal steps of human erythroid differentiation.
Cell Reports 2021 34 Gonzalez Menendez