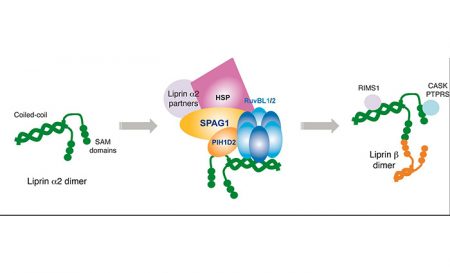
In a Nature Communications publication, Chloé Maurizy and colleagues (E. Bertrand’s team and 3 other teams from ANR consortium snoRNPASSEMBLY) have discovered a novel co-chaperone, called R2SP. It was known that macromolecular complex assembly requires dedicated machinery working with Hsp90. We and others have shown that R2TP/Hsp90 is involved in assembly of essential complexes such as snoRNPs, snRNPs, RNA polymerases and PIKKs. R2TP is composed of a RPAP3-PIH1D1 heterodimer associated to an heterohexamer of RUVBL1 and RUVBL2 AAA+ ATPases, which has the chaperoning activity of the complex. By RMN studies, we solved the tri-dimensional structure of RPAP3 Cterminal domain. This domain was also shown to bind in vitro and in vivo to RUVBL1/2.
We then performed systematic analysis of interactions and found that SPAG1, a paralog protein of RPAP3 also a bearing RPAP3-like Cter domain, directly binds PIH1D2 (paralog of PIH1D1). SPAG1 and PIH1D2 form with RUVBL1/2 a complex that we called R2SP. Functional analysis of R2SP had shown its implication in Liprin protein stabilization and assembly with its partners to form complexes that are specifically expressed in brain and testis.
This data are useful to understand the molecular mechanism of Hsp90/70-R2TP and R2SP in quaternary assembly of client macromolecular complexes. Dysfuntion in these processes are at the origin of human pathologies such as neurodegenerative ones (Alzheimer, Parkinson and Spinal muscular atrophy) or more rare pathologies such as Dyskeratosis Congenita or Retinitis Pigmentosa and ribosomopathies.