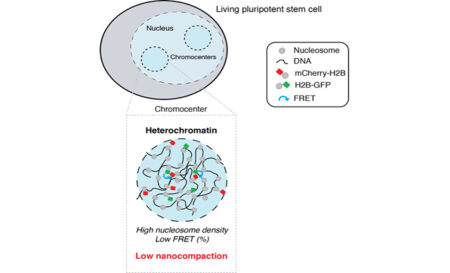
In this study published in EMBO Journal and supervised by David Llères and Robert Feil, using a FLIM-FRET microscopy imaging approach the group explored how chromatin, and in particular heterochromatin, is structured at the nanoscale in living murine embryonic stem cells (ESCs).
The majority of our genome is constituted of repeated DNA sequences that assemble into heterochromatin, a repressed chromatin state that is essential to constrain their mutational capability. Although repressive heterochromatin has been largely described to be a highly compacted, stable structure, most studies have been performed on fixed cells, often on transformed cells, or averaged from populations of millions of cells. It therefore remained unclear how heterochromatin is compacted at the nucleosomal level in living primary cells and how such nanoscale compaction is regulated. By measuring FRET between fluorophore-tagged histones H2B we analysed, in living cells, the spatial organisation of nanometre range proximity between nucleosomes, which we named nanocompaction. We find that, contrary to expectations, constitutive heterochromatin is not highly compacted at the nanoscale in living ESCs but resides in a low nanocompaction state that depends on the balance between HP1a et b, isoforms, the H4K20me2/3 marks, and the cell proliferation marker Ki-67 protein.
Evidence for low nanocompaction of heterochromatin in living embryonic stem cells. Dupont C, Chahar D, Trullo A, Gostan T, Surcis C, Grimaud C, Fisher D, Feil R*, Llères D*. EMBO J. 2023 Apr 21:e110286.
https://doi.org/10.15252/embj.2021110286.