The research of our group has focused on various aspects of human and murine T cell differentiation and responsiveness in the context of genetic and acquired immunodeficiencies as well as cancers. We have also pursued extensive studies on the role of nutrient transporters and nutrient utilization in hematopoietic stem cell differentiation, in general, and T cell effector function, in particular. Our strategy of combining fundamental and translational approaches has promoted our research efforts, bringing new insights in several different arenas:
1.Modulating T cell differentiation by thymus-targeted gene and cell therapies
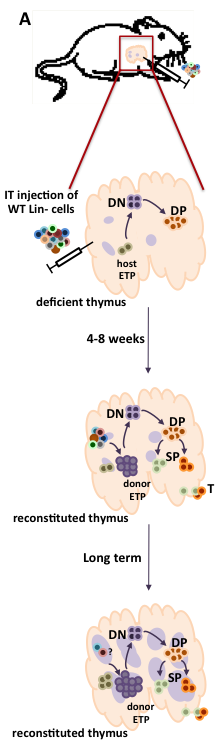
Hematopoietic stem cell transplantation (HSCT), the conventional therapy for patients with severe combined immunodeficiency, has a sub-optimal outcome in patients lacking histocompatible sibling donors. We have developed an intrathymic targeting approach to improve in vivo gene transfer and stem cell differentiation in a murine model of ZAP-70 deficiency (Adjali et al., PNAS, 2005; Adjali et al J Clin Invest, 2005) and have achieved high level intrathymic gene transfer in macaques (Moreau et al., Mol Therapy, 2009). These studies have also furthered our fundamental understanding of progenitor cell homing and lymphoid cell commitment, both in normal and SCID conditions and most notably, they have provided the first evidence that the thymus can, under certain conditions, support an autonomous differentiation (Vicente et al., Blood 2010; de Barros et al., Blood 2013; Stem Cells, 2013; Pouzolles et al., Curr Opin Hem, 2016; Figure 1).
Figure 1: Fate of intrathymic progenitor cell transplants in immunodeficient mice with available thymic niches.
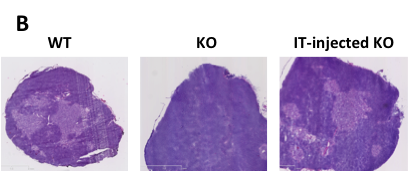
(A) WT hematopoietic progenitors, comprising lineage-negative (Lin-) cells, are intrathymically injected into immunodeficient mice with available thymic niches, such as that caused by ZAP-70-deficiency. These mice have only a rudimentary medulla (purple circles) and early thymocyte progenitors (ETP) are markedly reduced. Following intrathymic progenitor cell injection, thymopoiesis is restored by 4-8 weeks with increasing numbers of donor ETP as well as double negative (DN) immature progenitors, more mature CD4+CD8+ double positive (DP) thymocytes, as well as lineage committed CD4 and CD8 single positive (SP) thymocytes which emigrate from the thymus into the periphery. At long-term time points, the numbers of donor ETP remain elevated, fostering the continued differentiation of DP and SP thymocytes. (B) The direct intrathymic injection of hematopoietic progenitors into the thymus is associated with a long-term restoration of the thymic archicture. As shown in this hematoxylin-eosin staining of thymus sections from a WT, ZAP-70-KO (KO) and an IT-reconstituted ZAP-70-KO (IT-injected KO), intrathymic progenitor cell administration is associated with the presence of densely packed cortical regions and less dense medullary regions whereas KO mouse show defective medullary formation.
2.T cell reactivity and immunotherapy.
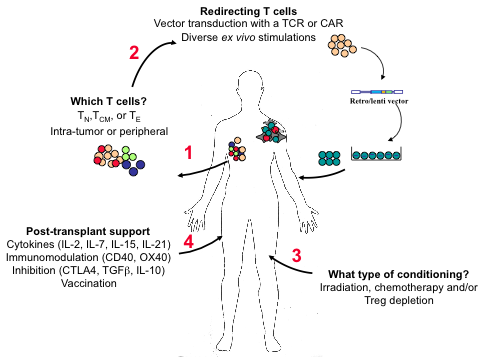
To improve immunotherapy strategies, the identification of factors and parameters that enhance the survival and reactivity of adoptively-transferred tumor-specific T cells is critical. Under lymphopenic conditions, antigen-specific CD4 cells are necessary to promote the differentiation of memory-like CD8 T cells into effectors with responsiveness to self antigen (Le Saout et al., PNAS, 2008; PLoS One, 2010) and more recently, we have found that the fate of adoptively-transferred T cells is conditioned by the lymphopenia-inducing regimen, due to differential effects on stromal and antigen-presenting cell subsets. These fundamental studies are being exploited for the utilization of CAR/TCR-engineered T cells in anti-tumor immunotherapy strategies (Figure 2). Moreover, in collaboration with Philippe Jay and colleagues, we found that Th2 differentiation in the small intestine is dependent on IL25 secretion by epithelial Tuft cells (Gerbe et al., Nature 2016).
Figure 2: Factors implicated in the success of adoptive T cell therapy (ACT) against tumors.
Important steps and critical questions in ACT are highlighted. It will be necessary to determine whether naïve (TN), central memory (TCM), or effector (TE) T cells are optimal for transfer and whether it is best to isolate them from peripheral blood or from the tumor itself (1). To obtain the most “fit” tumor-reactive T cells, a tumor-specific chimeric antigen receptor (CAR) or T cell receptor (TCR) can be introduced by retroviral- or lentiviral-mediated technology following ex vitro stimulation and/or expansion (2). These tumor-specific lymphocytes are then infused into tumor-bearing individuals preconditioned to favor the survival/persistence and activity of the transferred cells (3 and 4). Post-transplant treatment of the patients with cytokines, stimulatory or antagonist antibodies, and vaccination further favors the in vivo expansion of tumor-specific lymphocytes.
3.Metabolic regulation of T cell activation, differentiation and retroviral infection.
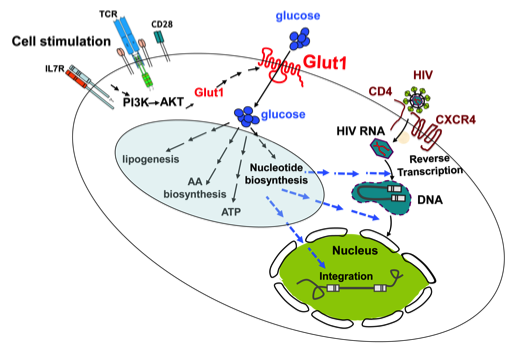
The discovery by Marc Sitbon and colleagues that the glucose transporter Glut1 is the receptor for the human T cell leukemia virus (Manel et al., Cell, 2003) has fostered our continued collaborative studies with their group in the IGMM, studying the role of this glucose transporter in T cell differentiation, activation and retroviral infection. We found that expression of Glut1 renders thymocytes and T cells significantly more susceptible to HIV infection (Figure 3; Loisel-Meyer et al., PNAS 2012) and our recent studies show that the differential use of glutamine and glucose conditions T cell differentiation and function. Furthermore, high Glut1 promotes effector cytokine secretion (Cretenet et al., Scientific Reports 2016) while glutamine deprivation promotes the conversion of naïve T cells to a suppressor fate (Klysz et al. Science Signaling 2015) and glutamine-derived CTP is required for efficient T cell activation, with mutations resulting in immunodeficiency (Martin et al., Nature 2014). The importance of metabolism in T cell activation is also highlighted by our recent studies showing that the resveratrol polyphenol alters the effector function of human T cells via changes in their bioenergetic homeostasis (Craveiro/Cretenet et al. Science Signaling, in press).
Figure 3: HIV infection is promoted by TCR/cytokine-mediated upregulation of nutrient transporters.
HIV infects CD4+ T lymphocytes via CD4 and the CXCR4/CCR5 co-receptors. Following this step, uncoating of the entering virion, reverse transcription, nuclear entry and integration of the viral DNA are critical for a productive infection. These steps are inefficient in quiescent T lymphocytes but TCR/cytokine stimulation results in a significantly higher level of infection. Differences between quiescent and activated T cells are numerous but one major change is the metabolic state of the cells. Upon activation, there is a cell surface upregulation of multiple nutrient transporters including the Glut1 glucose transporter via a PI3K/AKT-mediated pathway. The enhanced transport of glucose results in the upregulation of numerous metabolic processes (indicated in the blue circle). We and others have found that blocking glucose transport in T cells inhibits HIV-1 infection but the precise metabolic processes regulating HIV-1 infection remain to be determined. The possible steps wherein glucose metabolism can influence HIV-1 infection are presented as dashed arrows.
4.Regulation of hematopoietic stem cell differentiation by nutrient transporter expression and function.
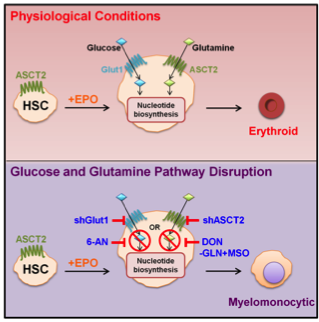
Hematopoietic stem cell (HSC) renewal and lineage differentiation are finely tuned processes, regulated by cytokines, transcription factors and cell-cell contacts. However, recent studies have shown that fuel utilization also conditions HSC fate. Intriguingly, the human erythrocyte is the cell type expressing the highest level of this transporter (>200,000 molecules/cell). Notably, we found that erythroid Glut1 expression is not a common trait across species but rather is restricted to those few mammals unable to synthesize ascorbic acid (AA). Indeed, Glut1 transports an oxidized form of vitamin C, L-dehydroascorbic acid (DHA), likely providing a compensatory mechanism for those species that have lost the ability to synthesize the essential amino acid metabolite (Montel-Hagen et al., Cell, 2008; Blood, 2008). Most recently, we have identified fuel resource availability as a critical regulator of HSC commitment and differentiation to distinct lineage fates. Glutamine fuels erythroid specification via nucleotide biosynthesis and blocking glutaminolysis diverts HSCs to the myelomonocytic lineage (Figure 4; Oburoglu et al., Cell Stem Cell, 2014; Oburoglu et al, Curr Opin Hem, 2016). These results support ongoing work on nutrient transport/ utilization as regulators of HSC development and suggest that aberrant metabolic reprogramming contributes to pathological lineage differentiation.
Figure 4: Lineage specification of HSCs to an erythroid lineage fate requires nucleotide biosynthesis.
Under physiological conditions, erythropoietin promotes erythroid differentiation of HSCs, a process requiring glucose and glutamine metabolism. Under conditions where glutaminolysis Is blocked by downregulation of the ASCT2 glutamine transporter or by drugs (DON, MSO), erythroid differentiation is blocked and cells progress to a myelomonocytic cell fate. Erythroid specification requires de novo nucleotide biosynthesis which is also provided by the glucose-fed pentose phosphate shunt and can be blocked by downregulating the glucose transporter Glut1 or the G6PD enzyme (via 6-AN).